
As a past surgeon for thirty seven years (37), I am a doctor who worked in the trenches in missionary work, in the operating rooms...

Simplified and Modified Explanation of Targeted Therapy to Treat Cancer (from NCI), (American Cancer Society) for public awareness and education about combined as “individualized precision modern approach” to cancer treatment Edited by George Yu MD 1/1/2023
Targeted therapy treats cancer by targeting proteins that control how cancer cells grow, divide, and spread.
What is targeted therapy?
Targeted therapy is a type of cancer treatment that targets proteins that control how cancer cells grow, divide, and spread. It is the foundation of precision medicine. As researchers learn more about the DNA changes and proteins that drive cancer, they are better able to design treatments that target these proteins. The TCGA cancer genome atlas did not show an uniform genome for each cancer as expected, but small breakthroughs came as a new discovery of mutations in certain cancers and this was the beginning of more targeted therapy.
How is targeted therapy different from chemotherapy?
Targeted therapy drugs, like other drugs used to treat cancer, are technically considered chemotherapy. But targeted therapy drugs don’t work the same way as traditional or standard chemotherapy (chemo) drugs. Targeted drugs zero in on some of the changes that make cancer cells different from normal cells. This makes them work differently from chemotherapy in two keyways:
What are the types of targeted therapy?
Most targeted therapies are either small-molecule drugs (generic name ends with “Nib”) or monoclonal antibodies (generic name ends with “Mab”.
Small-molecule drugs are small enough to enter cells easily so they are used for targets that are inside cells.
Knowing these details has led to the development of drugs that can “target” these proteins or enzymes and block the messages being sent. Targeted drugs can block or turn off signals that make cancer cells grow or can signal the cancer cells to destroy themselves.
Monoclonal antibodies (xxxx mab), also known as therapeutic antibodies, are proteins produced in the lab in large quantities. These proteins are designed to attach to specific targets found on cancer cells. Some monoclonal antibodies mark cancer cells so that they will be better seen and destroyed by the immune system. Other monoclonal antibodies directly stop cancer cells from growing or cause them to self-destruct. Still others carry drugs and toxins to cancer cells. Few well known monoclonal antibodies such as trastuzumab, pembrolizumab, and rituximab are used to treat cancer.
How targeted therapy works
Targeted therapies are made to find and attack specific areas or substances in cancer cells and it can detect and block certain kinds of messages sent inside a cancer cell that tell it to grow. Some of the substances in cancer cells that become the "targets" of targeted therapies are:
The action of targeted drugs can work to:
The action of the drugs can affect where these drugs work and what side effects they cause.
It's important to note that some targeted therapy drugs, for example, monoclonal antibodies, work in more than one way to control cancer cells and may also be considered immunotherapy because they boost the immune system.
Targeted therapy as precision medicine
Targeted therapy is sometimes called precision medicine or personalized medicine. This is because they are made to exactly target specific changes or substances in cancer cells, and these targets can be different even when people have the same type of cancer. Certain types of tumors are tested for different targets after a biopsy or surgery, and this can help find the most effective treatment. Finding a specific target makes matching patients with treatment more precise or personalized.
Some targeted drugs are more “targeted” than others. Targeted therapies are classified as either small or large molecule drugs.
Types of targeted therapy
Many kinds of cancer can be treated with targeted therapies, and there are many different types of targeted therapies. Here are some types with a few examples of how they are used.
Who is treated with targeted therapy?
For some types of cancer, such as chronic myelogenous leukemia (also known as CML), most people with that cancer will have a target for a certain drug, so they can be treated with that drug. But most of the time, your tumor will need to be tested to see if it contains targets for which there is a drug.
Testing your cancer for targets that could help choose your treatment is called biomarker testing. See Biomarker Testing for Cancer Treatment for more information.
You may need to have a biopsy for biomarker testing. A biopsy is a procedure in which your doctor removes a piece of the tumor for testing. There are some risks to having a biopsy. These risks vary depending on the size of the tumor and where it is located. Your doctor will explain the risks of having a biopsy for your type of tumor.
Look up your type of cancer on the list of targeted therapy drugs approved to treat specific cancers to learn more about drugs that may be an option for you.
How does targeted therapy work against cancer?
Most types of targeted therapy help treat cancer by interfering with specific proteins that help tumors grow and spread throughout the body. This is different from chemotherapy, which often kills all cells that grow and divide quickly. The following explains the different ways that targeted therapy treats cancer.
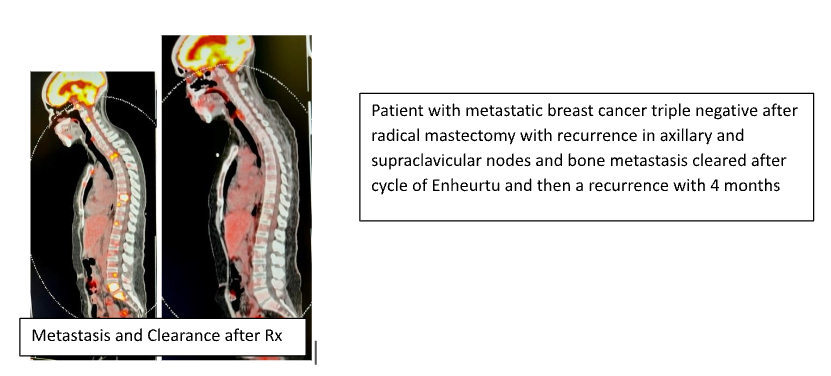
This patient has metastatic breast cancer and after Enheurtu treatment, she responded with a subsequent PET scan which showed complete cancer regression for the treatment but developed “Resistance” recurrence of the same lesions on the following scans. Generally, the therapy seems to develop resistance with recurrence within 3 to 8 months. Therefore, the importance of using a multi-prong approach including a mitochondrial metabolic nutritional ketone approach with metabolic blocking agents, with constant metronomic and micro-dose cytotoxic drugs, use of repurposed drugs such as metformin etc. Microsatellite instability (MSI) is the condition of genetic hypermutability or a predisposition to mutations in cells that results from the bodies impaired DNA mismatch repair (MMR) mechanism. DNA MMR is an essential function and the way the body naturally corrects errors that spontaneously occur during cell division associated DNA replication.
Microsatellite instability (MSI) is the condition of genetic hypermutability or a predisposition to mutations in cells that results from the bodies impaired DNA mismatch repair (MMR) mechanism. DNA MMR is an essential function and the way the body naturally corrects errors that spontaneously occur during cell division associated DNA replication.
Are there drawbacks to targeted therapy?
What are the side effects of targeted therapy?
When targeted therapy was first developed, scientists thought that it would be less toxic than chemotherapy. But they have learned that targeted therapy can also cause serious side effects. The side effects that you may have depends on the type of targeted therapy you receive and how your body reacts to it.
The most common side effects of targeted therapy include diarrhea and liver problems. Other side effects might include
Very rarely, a hole might form through the wall of the esophagus, stomach, small intestine, large bowel, rectum, or gallbladder.
There are medicines for many of these side effects. These medicines may prevent the side effects from happening or treat them once they occur.
Most side effects of targeted therapy go away after treatment ends.
How is targeted therapy given?
Small-molecule drugs are given orally and medications and Monoclonal antibodies are given intravenously
Only One Recent Success Story with a follow up of up to 24 month is the monoclonal antibody Dostarlimab anti PD-1 inhibitor ( NEJM June 5, 2022 , Cercek A - NCT04165772) Jemperli an anti-PD-1 monoclonal antibody, was reported to have a 100% clinical complete response up to 24 months among patients with mismatch repair-deficient locally advanced rectal cancer at the American Society of Clinical Oncology 2022 annual meeting
About Jemperli (Dostarlimab)
Jemperli (dostarlimab or TSR-042) is a humanized anti-programmed death (PD)-1 monoclonal antibody that binds with high affinity to the PD-1 receptor and effectively blocks its interaction with the ligands PD-L1 and PD-L2. Jemperli is a novel precision cancer immunotherapy drugs that helps to restore the body’s immune system in fighting cancer by releasing checkpoints that cancer uses to shut down the immune system. PD-1 and PD -L1 are proteins that inhibit certain types of immune responses, allowing cancer cells to evade an attack by the body’s immune cells. Dostarlimab works similar to other checkpoint inhibitors.
Microsatellite instability (MSI) is the condition of genetic hypermutability or a predisposition to mutations in cells that results from the bodies impaired DNA mismatch repair (MMR) mechanism. DNA MMR is an essential function and the way the body naturally corrects errors that spontaneously occur during cell division associated DNA replication.
Mismatch Repair Genes work like genetic “spell checkers.” When problems occur in these spell-checking MMR genes, it means that areas of DNA start to become unstable, and the body is unable to correct the errors that occur during DNA replication and consequently accumulate errors. The accumulation of errors causes the creation of novel microsatellite fragments that can be measured. The presence of MSI represents evidence that the MMR function is not working normally and predisposition to developing cancer exists.
Because Mismatch repair-deficient tumors are known to be sensitive to checkpoint inhibitor immunotherapy treatment researchers evaluated Jemperli in patients with early stage rectal cancer to see if it could replace radiation and surgery. Fourteen patients with mismatch repair-deficient stage II and stage III rectal adenocarcinoma were treated with Jemperli every 3 weeks for 6 months, followed by standard chemoradiation and surgery. However, those who achieved a complete response to treatment could omit chemoradiation and surgery. With median follow-up of 6.8 months Jemperli was found to induce a complete response in all 14 patients. If these patients maintain their response, they are effectively cured without having to undergo surgery and radiation therapy. This advance would allow patients with advanced stage rectal cancer to benefit from curative therapy without enduring the cost and side effects of surgery and radiation. Additional patients are enrolling in clinical trials to confirm the reported benefit of Jemperli in rectal cancer.